FDA Approves Ramucirumab Plus Erlotinib for First-Line Treatment of Metastatic NSCLC

On May 29, 2020, the U.S. Food and Drug Administration (FDA) approved ramucirumab (Cyramza®) in combination with erlotinib for first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 mutations.
- Read more about FDA Approves Ramucirumab Plus Erlotinib for First-Line Treatment of Metastatic NSCLC
- Add new comment
COVID-19 Challenges Showcase Stories of Nurses’ Strength

The Emotional Burden of COVID-19 Almost Made Me Leave Nursing

Life can steer you down a road that changes your impressions and view of the world. Without conscious awareness, it distorts comprehension and challenges you to change or be a byproduct of the times. Fighting to go back in time can destroy your life, livelihood, and career.
Oncology Nursing Society Announces Awardees of Nurses Innovate QuickFire Challenge in Oncology

In honor of Oncology Nursing Month, the Oncology Nursing Society (ONS) announced today the two awardees selected in the Johnson & Johnson Nurses Innovate QuickFire Challenge in Oncology. The Challenge was designed with the goal of accelerating innovation in improving oncology care, including prevention, early detection, treatment, and care for cancer survivors.
- Read more about Oncology Nursing Society Announces Awardees of Nurses Innovate QuickFire Challenge in Oncology
- Add new comment
Be Honest: Are You Getting Enough Sleep?

A full night’s sleep is a necessity, not a luxury, yet many people place sleep at the end of their priority list. Rather than seeing it as restorative, they think it takes up precious time to be productive. Many proudly proclaim, “You can sleep when you’re dead,” but ignoring healthy sleep habits can actually bring people closer to that end. Insufficient sleep is so pervasive in the United States that the Centers for Disease Control and Prevention considers it a public health epidemic.
FDA Approves Nivolumab Plus Ipilimumab and Chemotherapy for First-Line Treatment of Metastatic NSCLC

On May 26, 2020, the U.S. Food and Drug Administration (FDA) approved the combination of nivolumab plus ipilimumab and two cycles of platinum-doublet chemotherapy as first-line treatment for patients with metastatic or recurrent non-small cell lung cancer (NSCLC) with no epidermal growth factor receptor or anaplastic lymphoma kinase genomic tumor aberrations.
- Read more about FDA Approves Nivolumab Plus Ipilimumab and Chemotherapy for First-Line Treatment of Metastatic NSCLC
- Add new comment
Proton Therapy May Reduce Radiation’s Severe Side Effects
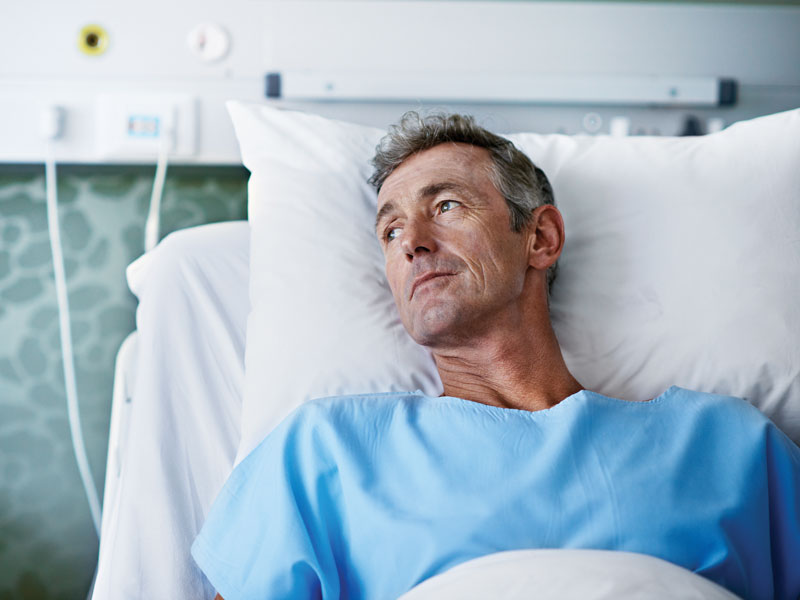
Proton beam radiation therapy is safer and equally effective as traditional radiation for adults with advanced cancers, researchers reported in study findings in JAMA Oncology.
FDA Approves Brigatinib for ALK-Positive, Metastatic NSCLC

On May 22, 2020, the U.S. Food and Drug Administration (FDA) approved brigatinib (Alunbrig®) for adult patients with anaplastic lymphoma kinase (ALK)-positive, metastatic non-small cell lung cancer (NSCLC). FDA also approved the Vysis ALK Break Apart FISH Probe Kit as a companion diagnostic for brigatinib.
Oncology Drug Reference Sheet: Capecitabine

Capecitabine (Xeloda®) was approved by the U.S. Food and Drug Administration (FDA) in 1998 as a nucleoside metabolic inhibitor with antineoplastic activity indicated for adjuvant colon cancer, metastatic colorectal cancer, and metastatic breast cancer.
This Oncology Nursing Month, Access the Value of ONS Local Chapters

In honor of Oncology Nursing Month this May, board members from five ONS chapters across the United States shared why ONS means so much to them.





