CMS Releases Report on Oncology Care Model

The cost of cancer care and the quality of patient services has always been a top priority in health care. However, the Centers for Medicare & Medicaid Services (CMS) Innovation Center has been working to strengthen both elements of care. CMS, through its Oncology Care Model (OCM) division, works with cancer care providers to develop payment strategies and performance categories in treatment plans for patients with cancer.
FDA Grants Accelerated Approval to Blinatumomab for B-Cell Precursor ALL

On March 29, 2018, the U.S. Food and Drug Administration (FDA) granted accelerated approval to blinatumomab (Blincyto®) for the treatment of adult and pediatric patients with B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1%.
- Read more about FDA Grants Accelerated Approval to Blinatumomab for B-Cell Precursor ALL
- Add new comment
Oncology Nurses Play Key Role in Genetics Education, Testing for Patients
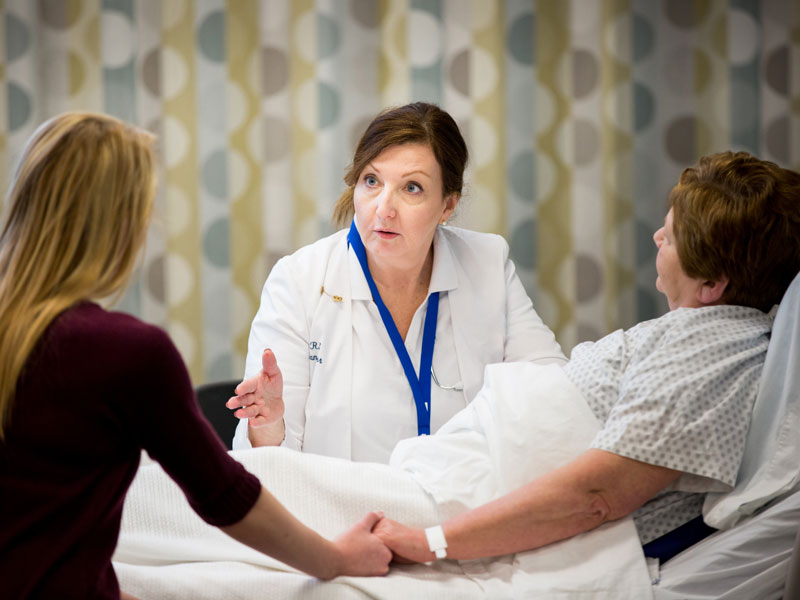
Identifying genetically predisposed women with breast cancer who could benefit from risk assessment and genetic counseling is an important competency for oncology nurses. However, a recent study published in the Journal of Clinical Oncology (JCO) reported that fewer than 50% of newly diagnosed patients with breast cancer who should have been given formal genetic counseling actually received the appropriate genetic testing.
- Read more about Oncology Nurses Play Key Role in Genetics Education, Testing for Patients
- Add new comment
Newly Approved Cancer Treatments Indicate Growing Role of Genomics and Oral Therapies
It is becoming more commonplace for nurses to find orders for agents with which they are unfamiliar or quite possibly have never administered. Following is a summary of the latest new U.S. Food and Drug Administration (FDA) approvals or indications to keep you up to date in your practice. Of note, this summary contains the approval of yet another biosimilar in trastuzumab-dkst and rolapitant for chemotherapy-induced nausea and vomiting, which includes a safety alert. Early experiences with rolapitant, a NK-1 inhibitor, indicated a risk of hypersensitivity reactions.
- Read more about Newly Approved Cancer Treatments Indicate Growing Role of Genomics and Oral Therapies
- Add new comment
Prevent Colorectal Cancer Through Screening

Of cancers affecting both men and women, colorectal cancer (CRC) is the second leading killer in the United States. In 2014, according to the Centers for Disease Control and Prevention, 139,992 people in the United States were diagnosed with CRC and 51,651 people died from it. Oncology nurses know that screening tests allow for healthcare providers to remove polyps before they become cancer or identify CRC in its earliest, most treatable stages. Clearly, screening is key to preventing CRC, most insurance plans cover screening, and patients now have more screening test options than ever. So why is CRC still so common? Why do people we know and care about still get this disease?
Lay Patient Navigators Can Help Oncology Nurses Guide Patients Through Cancer Care

Even for medical professionals working in health care every day, the U.S. healthcare system can be incredibly complex. Understanding where to obtain information and how to connect patients to resources can be difficult. For patients, navigating their treatment journey can be—at times—downright impossible. Coordinating care for patients with cancer is a crucial component to successful outcomes and quality cancer care.
- Read more about Lay Patient Navigators Can Help Oncology Nurses Guide Patients Through Cancer Care
- Add new comment
Managing Weight Loss in Patients With Cancer

Patients’ weight and nutrition status will often vary throughout the cancer care continuum. Weight loss might occur before the diagnosis, be one of the presenting cancer symptoms, be related to the tumor itself, or be secondary to side effects of their treatment (e.g., anorexia from chemotherapy or radiation).
Key Funding Increases for Cancer Research, Nursing, Public Health; Patients, Providers, or Politicians: Whose Choices Matter Most?; FDA Targets Flavored Tobacco Products

Racing against the clock to ensure the government stayed funded through September 2018, President Trump signed the Consolidation Appropriations Act, a $1.3 trillion spending bill that includes funding for a number of key nursing and public health initiatives. The bill, which had made its way through the House of Representatives and the Senate last week, also contains new clarifying language for the Dickey Amendment, ending a 22-year ban on government-funded gun violence research. ONS joined the Nursing Community Coalition—led in part by the efforts of the American Nurses Association—to support evidence-based inquiry into gun violence and its potential impacts on public health.
- Read more about Key Funding Increases for Cancer Research, Nursing, Public Health; Patients, Providers, or Politicians: Whose Choices Matter Most?; FDA Targets Flavored Tobacco Products
- Add new comment
ONS Membership Elects New Board of Directors
FDA Approves Nilotinib for Pediatric Patients With Chronic Phase Ph+ CML

On March 22, 2018, the U.S. Food and Drug Administration (FDA) approved nilotinib for pediatric patients 1 year of age or older with newly diagnosed Philadelphia chromosome positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) or Ph+ CML-CP resistant or intolerant to prior tyrosine-kinase inhibitor therapy.





