Live a Life in Balance

Although you may not always believe it, living a balanced life is not out of reach. Nurses have a variety of ways to achieve daily balance and well-being, both informally and through dedicated programs. Today, institutions and nursing organizations alike are prioritizing initiatives to support and strengthen nurses’ well-being.
First KRAS-Targeted Therapy Receives FDA Approval for Lung Cancer
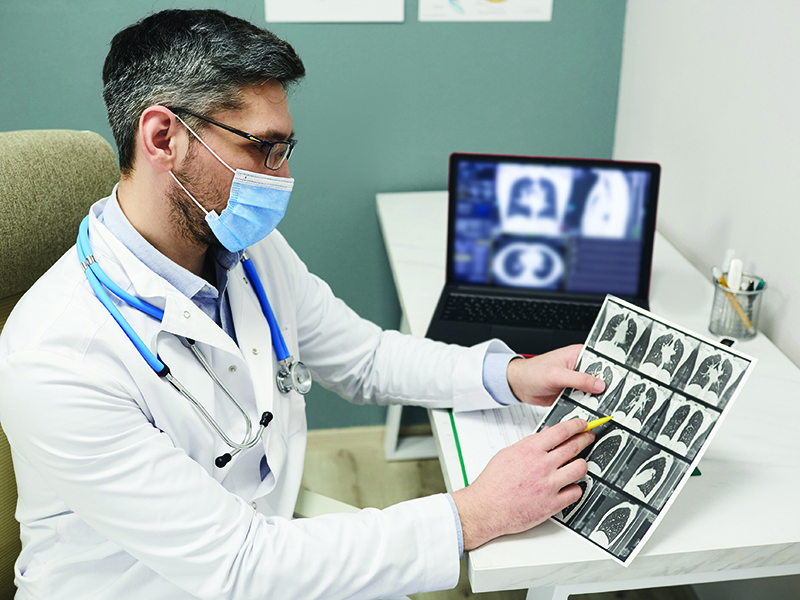
Sotorasib offers a durable clinical benefit without new safety signals in patients with previously treated KRAS p.G12C-variant non-small cell lung cancer, according to the results of a clinical trial that led to the therapy’s U.S. Food and Drug Administration (FDA) approval. The researchers reported the findings in the New England Journal of Medicine.
FDA Approves Sirolimus Protein-Bound Particles for Malignant Perivascular Epithelioid Cell Tumor

On November 22, 2021, the U.S. Food and Drug Administration (FDA) approved sirolimus protein-bound particles for injectable suspension (Fyarro™) for adult patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumor (PEComa).
- Read more about FDA Approves Sirolimus Protein-Bound Particles for Malignant Perivascular Epithelioid Cell Tumor
- Add new comment
Oncology Drug Reference Sheet: Sotorasib
After clinical trials demonstrated an overall response rate of 36% and median response duration of 10 months, the U.S. Food and Drug Administration granted sotorasib (LumakrasTM) accelerated approval on May 28, 2021, for the treatment of adults with KRAS G12C–altered locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test, who have received at least one prior systemic therapy.
Exercise the Evidence: How I Moved From an Idea to Program Development

As a clinical nurse specialist (CNS) with a cardiovascular background, I have seen the evidence-based benefits of exercise in a variety of settings. However, several years ago, when I was working as a CNS on an acute inpatient oncology unit, I noticed that exercise was not regularly included in care plans. As I learned from staff, this was done out of concern that patients needed to rest to save their energy.
- Read more about Exercise the Evidence: How I Moved From an Idea to Program Development
- Add new comment
New NIH Program Aims to Increase Gene Therapies for Rare Diseases

To accelerate the development of gene therapies for the 30 million Americans who have been diagnosed with a rare disease, in October 2021 the National Institutes of Health (NIH), U.S. Food and Drug Administration (FDA), 10 pharmaceutical companies, and five nonprofit organizations announced the creation of the Bespoke Gene Therapy Consortium (BGTC). The consortium is optimizing and streamlining the gene therapy development process to help fill the unmet medical needs of people with rare diseases.
Legislators Want Medicare to Negotiate Drug Prices to Improve Access and Affordability
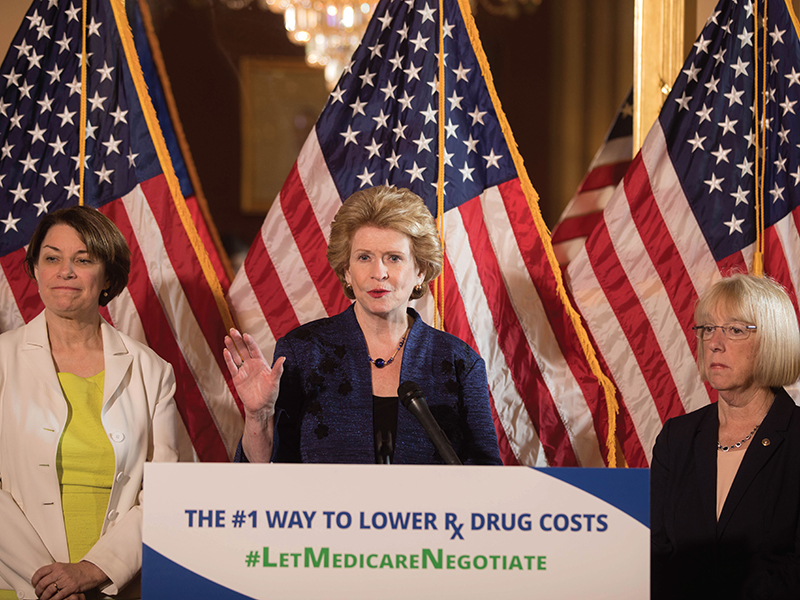
Few political and policy issues resonate with all Americans as much as the costs of prescription medications, particularly those for acute and chronic diseases that affect the body and spirit, like cancer. Seizing the opportunity for real change, in August 2021 President Joe Biden made a major announcement about his administration’s intent to change the dynamic on the financial impact of drugs and government oversight.
- Read more about Legislators Want Medicare to Negotiate Drug Prices to Improve Access and Affordability
- Add new comment
FDA Permits Marketing of E-Cigarette Products, Marking First Authorization of Its Kind

Under the authority given in the Family Smoking Prevention and Tobacco Control Act, in October 2021 the U.S. Food and Drug Administration (FDA) authorized the marketing of three new electronic nicotine delivery system (ENDS) products, “marking the first set of ENDS products ever to be authorized by FDA through the Premarket Tobacco Product Application pathway.” The agency made the announcement it continues its review of thousands of tobacco and e-cigarette products’ marketing applications.
- Read more about FDA Permits Marketing of E-Cigarette Products, Marking First Authorization of Its Kind
- Add new comment
Mid-Century Antibiotic May Offer Options After PARP Inhibitor Resistance

Novobiocin, an antibiotic discovered during the 1950s, may be a potential second-line therapy for patients whose tumors have become resistant to poly (ADP-ribose) polymerase (PARP) inhibitors, researchers reported in Nature Cancer. The finding may open up new options for patients with BRCA-variant disease such as breast, ovarian, pancreatic, and prostate cancer.
- Read more about Mid-Century Antibiotic May Offer Options After PARP Inhibitor Resistance
- Add new comment
Maryland Governor Announces Steps to Increase Nursing Workforce Statewide

Out-of-state RNs can now practice in Maryland and certain qualified nursing students are fast tracking graduation, according to Maryland Governor Larry Hogan’s (R-MD) September 2021 announcements. The Old Line State’s steps will help increase the workforce and curtail the nursing shortage.





