HHS Launches Network of Leaders and Organizations to Encourage COVID-19 Vaccinations
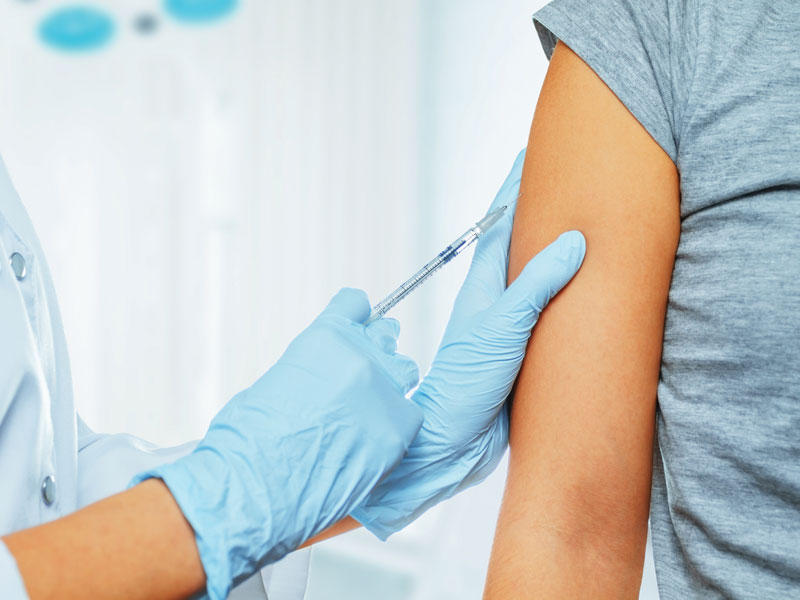
As of July 2021, more than 159 million individuals in the United States have been fully vaccinated against the COVID-19 coronavirus, totaling about 48.1% of the U.S. population. However, approximately 173 million others have not, or suggested they will not, receive the vaccination. President Biden’s goal of having 70% of Americans receive at least one vaccine dose and 160 million adults to be fully vaccinated against COVID-19 by July 4, 2021, fell short.
- Read more about HHS Launches Network of Leaders and Organizations to Encourage COVID-19 Vaccinations
- Add new comment
FDA Grants Regular Approval to Pembrolizumab and Lenvatinib for Advanced Endometrial Carcinoma

On July 21, 2021, the U.S. Food and Drug Administration (FDA) approved pembrolizumab (Keytruda®) in combination with lenvatinib (Lenvima®) for patients with advanced endometrial carcinoma that is not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), who have disease progression following prior systemic therapy in any setting, and are not candidates for curative surgery or radiation.
- Read more about FDA Grants Regular Approval to Pembrolizumab and Lenvatinib for Advanced Endometrial Carcinoma
- Add new comment
National Cancer Act Turns 50

Fifty years ago, President Richard Nixon delivered his third State of the Union address to the U.S. Congress, boldly outlining audacious goals with major federal funding attached. Seeking $100 million for what he deemed “the war on cancer,” Nixon pledged his commitment to invest federal resources in the fight against cancer.
FDA Announces Recall for 12 Lots of Varenicline Tablets Because of N-Nitroso Varenicline Content

On July 19, 2021, the U.S. Food and Drug Administration (FDA) reported Pfizer’s voluntary recall of two lots of varenicline 0.5 mg tablets, two lots of varenicline 1 mg tablets, and eight lots of a varenicline kit of 9.5 mg/1 mg tablets to the patient level because of the presence of N-nitroso-varenicline, a nitrosamine, above the Pfizer-established acceptable daily intake level. Varenicline is a treatment to help patients quit smoking and is intended for short-term use. To date, Pfizer has not received any reports of adverse events that have been related to the recall.
- Read more about FDA Announces Recall for 12 Lots of Varenicline Tablets Because of N-Nitroso Varenicline Content
- Add new comment
FDA Announces Recall of One Lot of Topotecan Injection Because of Presence of Particulate Matter

On July 1, 2021, the U.S. Food and Drug Administration (FDA) reported Teva Pharmaceuticals’ June 30, 2021, voluntary recall of lot 31328962B of topotecan injection 4 mg/4 ml. The recall was based on a report that a single vial contained a glass particle, a grey silicone particle, and a translucent, colorless cotton fiber.
- Read more about FDA Announces Recall of One Lot of Topotecan Injection Because of Presence of Particulate Matter
- Add new comment
Biden-Harris Administration’s Drug Policy Priorities Support Prevention, Treatment, and Recovery

Prescription overdoses and addiction rates have dramatically increased in the United States since 2012, with more than 70,000 deaths attributed to the abuse of fentanyl, opioids, cocaine, and methamphetamine in 2019, a 35% increase since 2015. Addressing drug misuse is a top priority for the Biden-Harris administration, as well as researching systematic inequities in the country’s approach to criminal justice and prevention, treatment, and recovery.
- Read more about Biden-Harris Administration’s Drug Policy Priorities Support Prevention, Treatment, and Recovery
- Add new comment
ONS Virtual Conference Offers Live and On-Demand Content for Oncology Nurses

This September, the Oncology Nursing Society (ONS) will host the second annual ONS Bridge™, the premier virtual conference that connects oncology nurses to resources and education covering two themes: care coordination and updates in therapy.
- Read more about ONS Virtual Conference Offers Live and On-Demand Content for Oncology Nurses
- Add new comment
Scientists Identify Protein Implicated in Tumor Growth, Treatment Resistance

A protein called AMBRA1 may be to blame for tumor resistance to CDK4/6 inhibitors, according to results from international teams of researchers that were reported in Nature.
- Read more about Scientists Identify Protein Implicated in Tumor Growth, Treatment Resistance
- Add new comment
Senate HELP Committee Reviews Federal Agencies’ COVID-19 Response

The Centers for Disease Control and Prevention (CDC) is “committed to continuing to advance the science around COVID-19, moving more vaccines into more communities—especially those communities most at risk for COVID-19 infection—and working to improve health equity,” CDC Director Rochelle Walensky, MD, MPH, said during a March 2021 U.S. Senate Committee on Health, Education, Labor, and Pensions (HELP) hearing. She joined Anthony Fauci, MD, David Kessler, MD, and Peter Marks, MD, PhD, in sharing witness testimonies about their agencies’ response to the pandemic and how to better prepare for future threats.
The Case of the Mysterious Myalgia

Randi is a 57-year-old patient who identifies as female. She was diagnosed with clear cell metastatic renal cell carcinoma (mRCC), and her past medical history includes mild hypertension managed with amlodipine and a two-year history of transient musculoskeletal pain managed with tramadol. She reports a family history of cardiovascular disease and rheumatoid arthritis (RA). Her primary care physician suspects Randi is at the beginning stages of fibromyalgia but has not made a conclusive diagnosis because she hasn’t experienced additional symptoms.





