kdonahue
elieditor
Katherine Leverence
Provider Groups Announce Campaign Urging Public to #WearAMask

Wearing a mask is critical to slowing the spread of the COVID-19 coronavirus pandemic, but the public hasn’t always been compliant. In an effort to provide education and understanding, the American Nurses Association, American Medical Association, and American Hospital Association joined forces to promote a new public health awareness campaign.
ONS Awards Celebrate Excellence in Cancer Care

Exemplary oncology nurses stand behind all of cancer’s treatments, supporting patients through side effects, shouldering their burdens and concerns, and advocating for quality care. Each year with its series of awards, the Oncology Nursing Society (ONS) recognizes just a few of the outstanding oncology professionals who have dedicated their lives to patient care.
FDA Study Reveals Higher COVID-19 Death Rate for Patients With Cancer

The U.S. Food and Drug Administration’s (FDA’s) Oncology Center of Excellence confirmed that those with immunocompromised systems, including cancer, are at greater risk for serious outcomes or death after contracting the COVID-19 coronavirus.
- Read more about FDA Study Reveals Higher COVID-19 Death Rate for Patients With Cancer
- Add new comment
Early Use of Immunotherapy Has Better Outcomes for Bladder Cancer

Starting immunotherapy for bladder cancer shortly after initial treatment with chemotherapy is better than taking an extended break from cancer treatment, according to results from a study reported at the American Society of Clinical Oncology annual meeting.
Acknowledge and End Unequal Representation in Cancer Research and Improve Access to Care
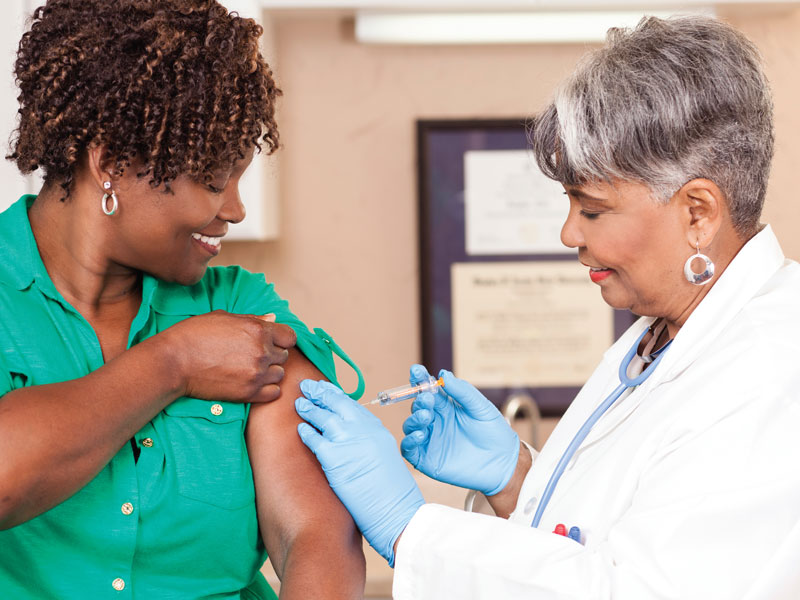
Research influences care along every inch of the cancer continuum, from prevention to survivorship, enabling healthcare professionals and patients to share decisions that result in the most current and tailored care strategies. It’s a powerful tool that sets the groundwork for providing optimal health outcomes. However, we must work to eliminate unequal representation.
- Read more about Acknowledge and End Unequal Representation in Cancer Research and Improve Access to Care
- Add new comment
FDA Approves First Liquid Biopsy NGS Companion Diagnostic Test for NSCLC

On August 7, 2020, the U.S. Food and Drug Administration (FDA) approved the Guardant360 CDx assay as the first liquid biopsy companion diagnostic that also uses next-generation sequencing (NGS) technology to identify patients with specific types of mutations of the epidermal growth factor receptor gene in patients with metastatic non-small cell lung cancer (NSCLC). This is the first approval to combine two technologies in one diagnostic test to guide treatment decisions.
- Read more about FDA Approves First Liquid Biopsy NGS Companion Diagnostic Test for NSCLC
- Add new comment
FDA Program Shares PROs From Cancer Clinical Trials

Cancer clinical trials often collect patient-reported outcome (PRO) data, but the information is generally used just for that trial. Recognizing the value of making it available to healthcare providers everywhere, in July 2020, the U.S. Food and Drug Administration (FDA) launched Project Patient Voice, a pilot program designed to share clinical trial PROs on an easy-to-access website.





