- Treatment Side Effects (http://dev-voice.ons.org/topic/treatment-side-effects)
- Patient Quality of Life (http://dev-voice.ons.org/topic/patient-quality-of-life)
- Radiation Therapy (http://dev-voice.ons.org/topic/radiation-therapy)
- Combination Therapies (http://dev-voice.ons.org/topic/combination-therapies)
The Case of Concurrent Therapy Concerns
By Elizabeth A. Strand, MSN, APRN-BC, and Kristin M. Ferguson, DNP, RN, OCN®
Sharon is a 60-year-old woman with metastatic breast cancer that was originally diagnosed in 2005 and treated with a mastectomy, deep inferior epigastric artery perforator flap reconstruction, chemotherapy, postmastectomy radiation, and five years of tamoxifen. Three months ago, her breast cancer recurred, and staging scans demonstrated metastatic disease in the lungs, left axilla, liver, and left iliac bone. A biopsy of the left iliac bone was ER positive, PR negative, and HER2 negative. Sharon began treatment with radiation to the painful left hip and letrozole, with the plan to start palbociclib once radiation was completed.
Sharon calls the on-call advanced practice provider over the weekend to report a “rash” on her left buttock, which she first noticed four days earlier. She describes it as itchy and tender with enlarging erythema and a feeling like the skin may have been punctured by a foreign object. She also describes new blisters forming with nonpurulent drainage.
What Would You Do?
The use of CDK4/6 (https://pubmed.ncbi.nlm.nih.gov/26947331/) inhibitors (palbociclib with letrozole (https://www.nejm.org/doi/full/10.1056/NEJMoa1607303) or fulvestrant) is the current standard of care for first-line, estrogen-positive, HER2-negative breast cancer. The regimen has provided an effective treatment with minimal toxicity and improved quality of life for many patients with metastatic disease, often enabling them to avoid chemotherapy for several years.
Many patients with metastatic breast cancer require additional palliative radiation in combination with the agents. The synergistic effects and safety of those therapies is currently not well documented (https://pubmed.ncbi.nlm.nih.gov/31360799/) in the literature. Whereas the most common side effect of palbociclib is neutropenia, we are continuing to learn (https://www.redjournal.org/article/S0360-3016(19)33234-1/fulltext) more about potential side effects when used in combination with palliative radiation. Newer oral therapies, specifically palbociclib, have been associated with increased grade 2–3 skin toxicity (https://www.nature.com/articles/s41416-020-0957-9) (radiodermatitis (https://www.ons.org/podcasts/episode-128-manage-treatment-related-radiodermatitis-ons-guidelinestm)) when used concurrently (https://pubmed.ncbi.nlm.nih.gov/30948930/) or within 14 days of radiation.
Skin toxicity is a common side effect of radiation. Most cases can be treated (https://www.ons.org/articles/ons-guidelinestm-cancer-treatment-related-radiodermatitis) with moisturizers, heal with time, and don’t require further intervention. Patients are educated to observe their skin, use lotions and emollients as needed, and to call with fever, pain, or protracted healing.
Currently, no prospective studies have been published on the safety of radiation treatment with palbociclib. A few retrospective studies (https://pubmed.ncbi.nlm.nih.gov/31360799/) have investigated the combination (https://www.nature.com/articles/s41416-020-0957-9), but they are (https://pubmed.ncbi.nlm.nih.gov/31100573/) too small to be definitive. Nurses and medical colleagues in both radiation and medical oncology must work together to educate and assess patients for complications of skin toxicity and be aware that the treatment combination may increase the likelihood of radiodermatitis (https://www.ons.org/articles/ons-guidelinestm-cancer-treatment-related-radiodermatitis), which has occurred both earlier than expected (https://www.nature.com/articles/s41416-020-0957-9) as well as weeks after (https://pubmed.ncbi.nlm.nih.gov/30948930/) radiation completion.
More prospective studies with larger cohorts are needed to answer some of the questions regarding who develops skin toxicity and whether timing of palbociclib or holding palbociclib for a set time around radiation makes sense. No standard exists of whether to hold the drug and, if so, for how long.
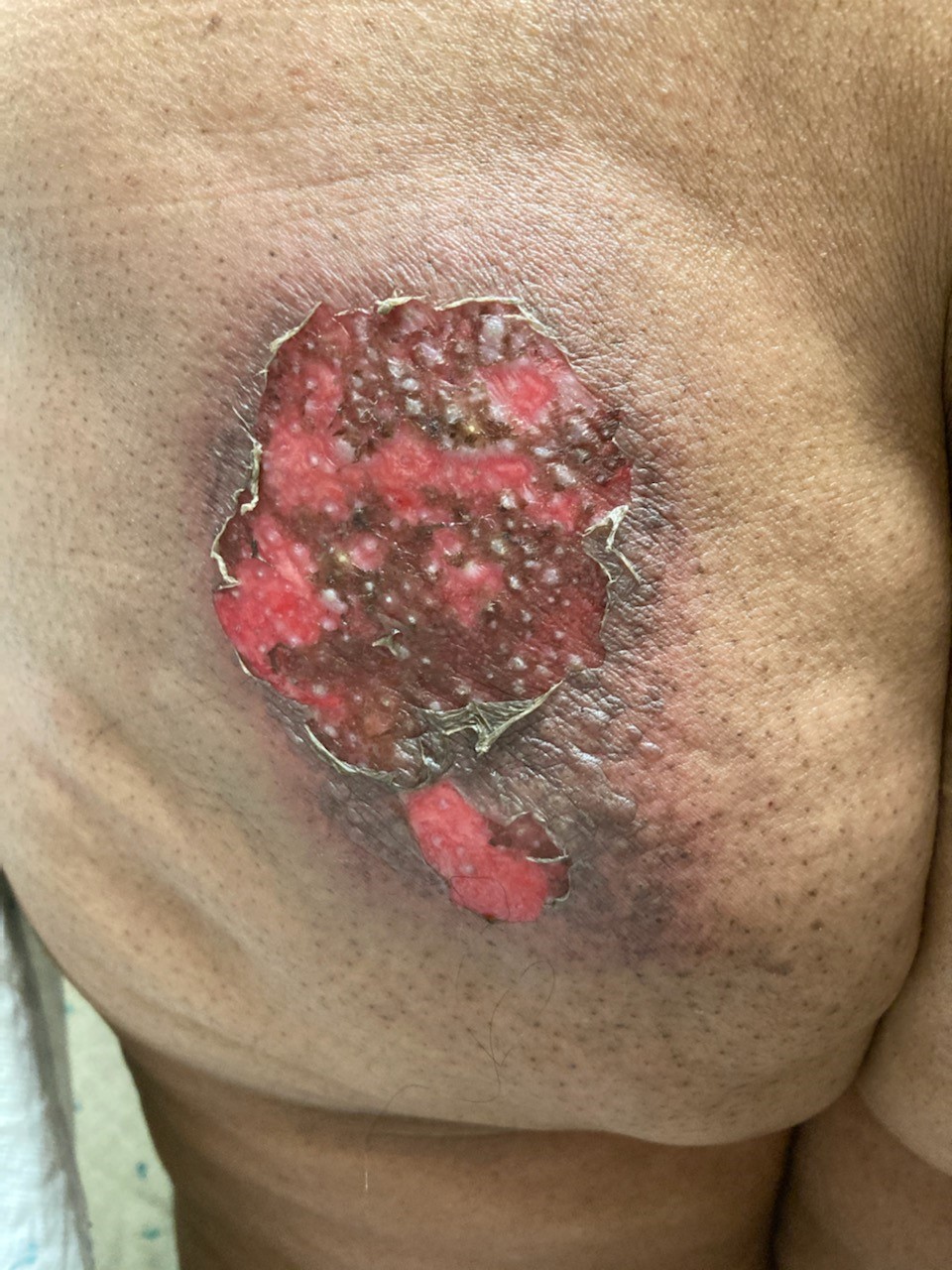
Sharon’s skin reaction developed approximately five weeks after radiation completion. The week before, cycle 2 of palbociclib was held because her absolute neutrophil count (ANC) was 600. She did not report any skin discomfort, rash, or skin changes prior to her weekend call.
After Sharon called, she sent a photo of the site and you prescribe clindamycin 300 mg four times a day for seven days for presumed cellulitis. She visited the medical oncology clinic four days after starting the antibiotic, and you rechecked her labs and assessed her skin. She reports less pain in the site, and her ANC has increased to 1,400. You examine her skin, which demonstrates a 9 cm x 6 cm area of diffuse erythema with areas of moist desquamation in the radiation field.
As her nurse practitioner, you consult with her radiation oncologist and confirm that the skin reaction is in the left iliac crest radiation portal. Sharon’s radiation team prescribes silver sulfadiazine cream to the area and reports that the site would likely take one to two weeks to heal.
The healthcare team delays cycle 2 of palbociclib by an additional two weeks. Sharon resumes the cycle after resolution of her left buttock skin toxicity.
This case highlights the importance of interdisciplinary oncology communication, patient education, skin examination of the radiation site up to six weeks post radiation, and careful review of a patient’s history. Radiation and medical oncology nurses, residents, and fellows are often the frontline patient educators as well as the providers who triage phone calls with patient complaints and must be aware of potential skin complications to better care for patients.
