Lohxa, LLC, issued a voluntary recall of five lots of its alcohol-free chlorhexidine gluconate oral rinse USP 0.12% because of a risk that the product may be contaminated with the bacteria Burkholderia lata. The U.S Food and Drug Administration (FDA) announced the recall on November 9, 2020.

“Use of the defective product in the immunocompetent host may result in oral and, potentially, systemic infections requiring antibacterial therapy,” the company reported. “In the most at-risk populations, the use of the defective product may result in life-threatening infections, such as pneumonia and bacteremia. To date, no adverse events have been reported to Lohxa, LLC, related to this recall.”
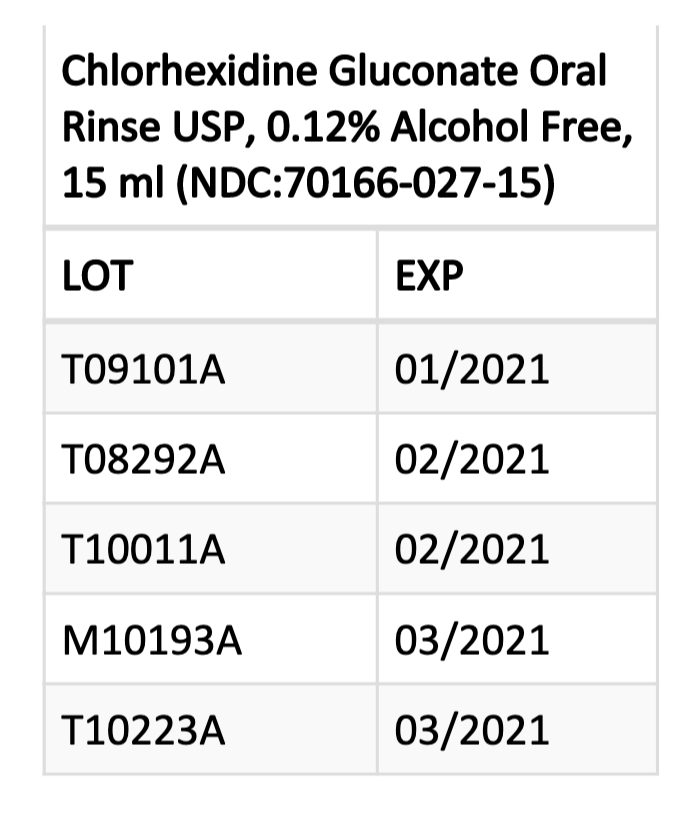
The prescription oral rinse product, available through institutional use only, is indicated for use as part of a professional program for the treatment of gingivitis. The affected product was distributed to hospital pharmacies across the United States in cases containing 50-unit dose cups. Each case has a colored label around the lid and body of the case.
Patients, pharmacies, and healthcare facilities in possession of the affected products (see chart) should stop using and dispensing them immediately.
Consumers with questions regarding the recall can contact Lohxa, LLC, at 800-641-5564 or email info@lohxa.com on Monday–Friday from 8 am–5 pm EST. They should contact their physician or healthcare provider if they have experienced any problems that may be related to using the product.
Healthcare providers should report adverse reactions or quality problems experienced during use of the product to FDA’s MedWatch Adverse Event Reporting program either online or by mailing or faxing a reporting form.
“Lohxa, LLC, is committed to delivering safe, fully compliant products of the highest quality and is taking necessary steps to prevent future occurrence of this issue,” the company said.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.





